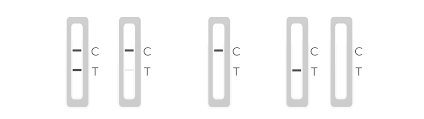
Description
![]()
Instructions for Use Healthcare Providers
INDICAID 3 month expiration extension
Regulatory Status: FDA EUA Authorized – this test has not been FDA cleared or approved but has been authorized by the FDA under an EUA for use by authorized laboratories.
Contents: Each box contains: Test Devices (2), Buffer solution (2), Nasal swabs (2), Package Insert (1) Quick Reference Instructions (QRI) (1)
Determinations: Detection of the SARS-CoV-2 nucleocapsid protein antigen
Storage Requirements: 2° – 30° C
Processing Time: 20 Minutes
4 Steps. 20 Minutes. 0 Discomfort.
Intended Use
The INDICAID COVID-19 Rapid Antigen At-Home Test is intended for non-prescription use with self-collected anterior nasal (nares) swab samples from individuals aged 14 or older, or an adult lay user testing another aged 2 years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 6 days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests. All negative results are presumptive. The INDICAID COVID-19 Rapid Antigen At-Home Test is only for in vitro diagnostic use under the Food and Drug Administration’s Emergency Use Authorization. This product has not been FDA cleared or approved.
*Covid tests are non-returnable.
See product links above for additional information or contact your local Stat Technologies sales representative