Description
![]()
![]()
FAQ Patients
FAQ Providers
FDA EUA letter
Indicaid Fact Sheet for Patients
Indicaid Fact Sheet for Providers
Instructions for use
Package Insert
INDICAID 6mo Shelf-life Extension
Regulatory Status: FDA EUA Authorized – this test has not been FDA cleared or approved but has been authorized by the FDA under an EUA for use by authorized laboratories. CLIA WAIVED
Contents: Each box contains: Test Devices (25), Buffer solution (25), Nasal swabs (25), Package Insert (1) Quick Reference Instructions (QRI) (1)
Determinations: Detection of the SARS-CoV-2 nucleocapsid protein antigen
Storage Requirements: 2° – 30° C
Processing Time: 20 Minutes
The INDICAID™ COVID-19 Rapid Antigen Test is a non-invasive rapid point-of-care diagnostic test for the qualitative detection of SARS-CoV-2 antigen in respiratory specimens. Each INDICAID™ COVID-19 Rapid Antigen Test is single-use and can analyze one anterior nasal swab sample. The total time required to perform one test is approximately 20 minutes from clinical specimen collection to result.
The INDICAID™ COVID-19 Rapid Antigen Test is a type of test called an antigen test. Antigen tests are designed
to detect proteins from the virus that causes COVID-19. SARS-CoV-2 infection, such as in individuals without known exposures to SARS-CoV-2 or residing in communities with low prevalence of infection. Your
healthcare provider will work with you to determine how best to care for you based on your test result(s) along with your medical history, and your symptoms.
- Nasal swab specimens may be self-collected by the patient if the collection procedure is instructed and observed by a healthcare professional.
• Process the collected specimen immediately after collection.
• Use only the swab provided in the INDICAID™ COVID-19 Rapid Antigen Test
Kit.
Internal Quality Control
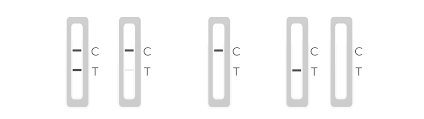
The INDICAID™ COVID-19 Rapid Antigen Test Device contains an internal procedural control to ensure that the test is functioning properly. The control line (C) on the Test Device will appear as a red-colored line and should appear
regardless of the test result. If the control line does not develop within 20 minutes, the test result is considered invalid and retesting should be performed with a newly collected sample, new Buffer Solution Vial, and a new Test Device.
The Indicaid COVID-19 Ag is intended for use by medical professionals or operators trained in performing tests
in point of care settings. The Indicaid COVID-19 Ag is only for use under the Food and Drug Administration’s
Emergency Use Authorization.
See product links above for additional information or contact your local Stat Technologies sales representative