Description
![]()

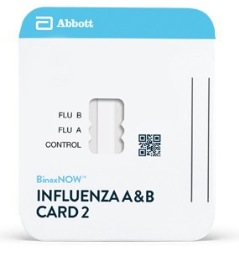
Unit: 1 Kit (22 Tests)
Regulatory Status: CLIA Waived/Professional Use Only
Contents: (22) Test devices, (25) Transfer Pipettes, (22) Elution Solution Vials, (22) NP Swabs, (1) Negative Control Swab, (1) Positive Control Swab, (1) Product Insert, (1) Procedure Card.
Storage Requirements: 35° – 86° F
Processing Time: 15 minutes
BinaxNOW Influenza A & B Card 2 With DIGIVAL Brochure US (English)
BinaxNOW™ Influenza A & B Card 2 is an in vitro immunochromatographic assay for the qualitative detection of influenza A and B nucleoprotein antigens in nasopharyngeal (NP) swab and nasal swab samples. It is intended to aid in the rapid differential diagnosis of influenza A and B viral infections. BinaxNOW™ Influenza A & B Card 2 must be read by the DIGIVAL™.
The first rapid antigen influenza test to achieve 510(k) clearance as a Class II assay under the new FDA Reclassification performance requirements. DIGIVAL provides objective result interpretation.
- Flexible sample types – Option of a Nasal Swab or Nasopharyngeal Swab sample type.
- Rapid results – Control of a connected instrument with the speed of a rapid lateral flow assay.
- Objective results – Eliminate operator subjectivity and establish consistency in the reading.
- Enhanced quality control – Quality Control module and a QC lockout feature to ensure quality controls are tested.
- Easy-to-use – Straight forward rapid assay procedure, followed by DIGIVAL interpretation.
- Workflow flexibility – Read Now Mode or Walk Away Mode to suit your workflow and throughput.
- Throughput – Interpret up to 30 assays per hour*
- Result management – Capture results on DIGIVAL, print them or share them through our Bi-Directional Connectivity