Description
![]()
![]()
Regulatory Status: CLIA Waived / Professional Use Only
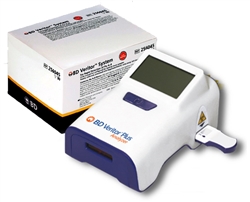
Contents: (1) Veritor™ Plus Analyzer, (2) Veritor™ Flu A+B Kits
The BD Veritor™ Plus System enhances point-of-care testing for SARS-CoV-2,* Flu A+B, RSV (respiratory syncytial virus), and Group A Strep assays, providing rapid diagnostic testing in a convenient, portable instrument.
The BD Veritor™ Plus System moves at the speed of what matters – you and your patients. The system offers what your organization needs to conduct rapid diagnostic antigen testing for respiratory infections.
The faster you know which infection is present, the faster you can respond. The BD Veritor™ Plus System puts the power of rapid diagnostic testing where you need it; easily, simply and effectively. The system’s rapid antigen testing assays detect respiratory pathogens, including SARS-CoV-2*, Flu A + B, Group A Strep, and Respiratory Syncytial Virus (RSV). See how the system supports your needs and adds value to your organization.
The BD Veritor™ System for Rapid Detection of Flu A+B is a rapid chromatographic immunoassay for the direct and
qualitative detection of influenza A and B viral nucleoprotein antigens from nasal and nasopharyngeal swabs of symptomatic
patients. The BD Veritor System for Rapid Detection of Flu A+B (also referred to as the BD Veritor System and BD Veritor
System Flu A+B) is a differentiated test, such that influenza A viral antigens can be distinguished from influenza B viral
antigens from a single processed sample using a single device. The test is to be used as an aid in the diagnosis of influenza
A and B viral infections. A negative test is presumptive and it is recommended that these results be confirmed by viral
culture or an FDA-cleared influenza A and B molecular assay. Outside the U.S., a negative test is presumptive and it is
recommended that these results be confirmed by viral culture or a molecular assay cleared for diagnostic use in the country
of use. FDA has not cleared this device for use outside of the U.S. Negative test results do not preclude influenza viral
infection and should not be used as the sole basis for treatment or other patient management decisions. The test is not
intended to detect influenza C antigens.