Description
Unit: 1 Box
Regulatory Status: CLIA Waived / Professional Use Only
Contents: (1) Assure Platinum Blood Glucose Meter, (1) User Instruction Manual
Storage Requirements: 32° – 122° F
Sample Volume: 5 uL
Processing Time: 5 seconds
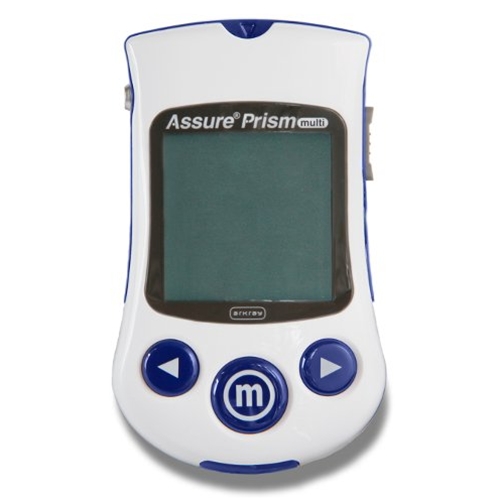
The Assure® Prism multi Blood Glucose Monitoring System gives healthcare professionals a fast, accurate and easy way to monitor their patients blood glucose levels with a clinically driven design and its advanced technology. With this monitor you will appreciate the single strip operation, quick test time, small blood sample and most of all its reliable accuracy.
This multi-resident meter complies with ISO 15197:2013 accuracy requirements. FDA approved for multi-patient use.
Assure Prism Features:
- Test strip that points toward the resident and backlit display are designed to make it easier for the healthcare professional to monitor a resident’s blood glucose level
- Fast test results in five seconds help reduce downtime
- Optimized readout display with large, thick numbers are intended to improve readability
- Small, compact, lightweight design provides better portability
- 20-600 mg/dL meter range
- 500 test memory with time and date stamp
- 7, 14, 30 and 90 day averaging
- Plasma referenced Results
- Auto code calibration
The Assure® Prism multi Blood Glucose Monitoring System is intended for the quantitative measurement of glucose in fresh capillary whole blood samples drawn from the fingertips and alternative sites such as the forearm, palm, thigh and calf. Alternative site testing should be used only during steady-state blood glucose conditions. The system is intended for use outside the body (in vitro) and is intended for multiple-patient use in a professional healthcare setting, as an aid to monitor the effectiveness of diabetes control. This system is only to be used with an auto-disabling, single-use lancing device. It is not intended for use on neonates and is not intended for the diagnosis or screening of diabetes.